
Rotavirus vaccines could help millions of African children survive and grow
Childcare, Early childhood development, Health and nutrition, Safe pregnancy and birth
A heat-proof vaccine being trialled in Niger could help to save 500,000 children each year and allow millions to grow and develop.
The United States has saved $1.2 billion in medical bills by vaccinating young children against the rotavirus, according to a new study.
But the vaccines, which prevented more than 380,000 children being hospitalised over five years, are expensive and need to be kept in a chilled environment.
That’s a challenge for sub-Saharan nations.
Now a new cheaper vaccine called BRV-PV that doesn’t need to be kept cold and has a shelf-life of a year is being trialled in Niger by Médecins Sans Frontières (MSF) with amazing results.
It could be the key to saving the lives of 500,000 children under five who die each year from dehydration and complications of rotavirus, particularly in sub-Saharan Africa.
Health is one of the five key areas for children under five if they are develop and fulfil their potential. That’s why Theirworld’s #5for5 campaign has been campaigning for world leaders to invest in early childhood development.
Children who survive rotavirus can have their development affected by diarrheoal disease. An infected intestine can fail to absorb important nutrients, which in turn can stunt a child’s growth.
Clinical Trial Director Dr Rebecca Grais said: “The results of the trial are very good. First and foremost, the vaccine is safe but more importantly we have found that it works.
“Behind this is 10 years’ work by a dedicated team in the field of treating patients and working on the study for a very long period of time.
“One of the reasons we decided to test this particular vaccine in Niger was because its profile was appropriate for the population where we work. The vaccine is heat-stable and is at a price that we hope will be sustainable for governments in the future to purchase.
“The first step is that it will be licensed and pre-qualified by the World Health Organisation (WHO) which will allow it to be widely used across Africa.”

In America, the rotavirus vaccine programme has stopped hundreds of thousands of children contracting the virus in the first place.
While the US study published in the Journal of the Pediatric Infectious Diseases Society reports how much money has been saved, the overall savings are likely to be far greater if other considerations, such as fewer doctors’ office or emergency room visits are included.
“Our findings confirm the sustained impact and effectiveness of the rotavirus vaccine programme,” study author Dr Eyal Leshem, who was working with the US Centers for Disease Control and Prevention at the time of the study.
It is hoped the new BRV-PV vaccine will not only save governments money but give the under-twos a better chance of survival. At the moment, 50% of all children under two contract the virus.
The new vaccine is also adapted to the type of rotavirus most commonly found in sub-Saharan countries and is safe.
An MSF spokesman said: “It is also affordable, with a price of under $2.50, which is much cheaper than the lowest price of rotavirus vaccines currently available. This should allow it to be rolled out quickly as part of routine immunisation programmes.”
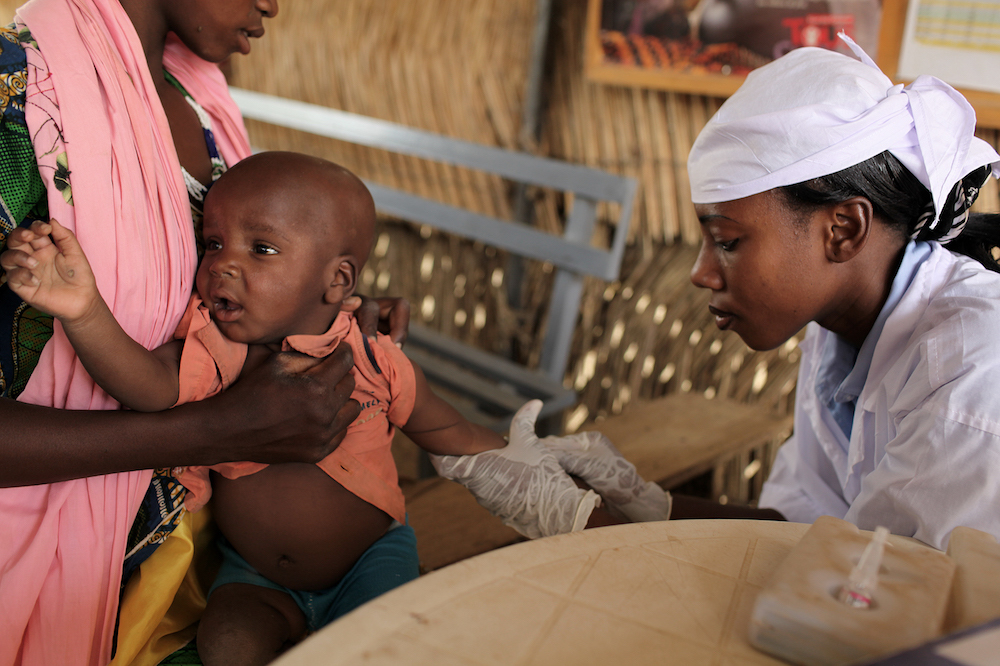
The trial was carried out on 4000 children under two years old. Results continued to show the vaccine had no safety concerns and had been proven to be as effective against severe gastroenteritis as the existing more expensive vaccines.
“This is a game-changer,” said MSF’s Dr Micaela Serafini, MSF Medical Director. “We believe that the new vaccine can bring protection against rotavirus to the children who need it most.
“The success of this trial shows that research and development into vaccines that are specifically adapted for use in low-income countries yields results.
“The quicker this vaccine is prequalified by the WHO, the sooner it can be used to prevent the deaths of thousands of children in the countries where it is needed most.”
The BRV-PV vaccine is currently under review by the WHO for pre-qualification. Once approved, low-income countries will be able to get the vaccine at an affordable price and roll it out.
More news

